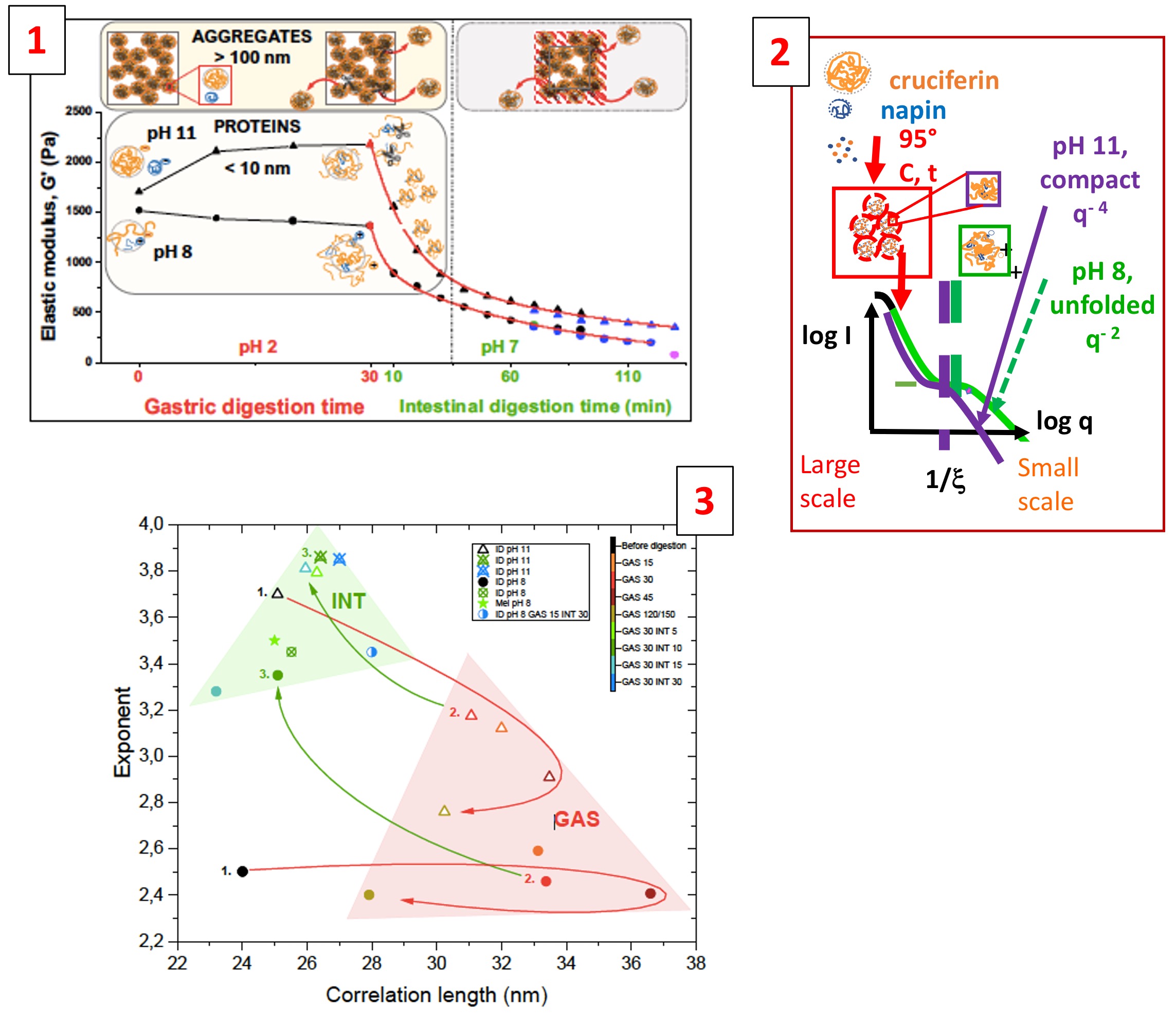
– Fig.1: The evolutions under gastric digestion of the G’ and G” moduli at ω = 1 s-1 show surprising differences for gels synthesized at two pHs (above or below the pI). Fig.2: The conformations after heating before digestion remain folded for pH11 while they are strongly unfolded at pH8. Fig.3: From the large q range variation of the exponent and of the correlation length ξ, we can monitor the state of unfolding / refolding and then scission, in GAStric then INTestinal phase. From the low q range variation, we try to infer the contribution of some aggregates –
We have studied the evolutions of rheological properties and nanostructures using small angle neutron scattering (SANS) of canola seed protein gels, containing cruciferin and napin, obtained by heating protein solutions prepared at pH 8 and pH 11. We focused on the gastric and the intestinal digestion of samples with dimensions of about ten millimeters, aiming at mimicking conditions of the human gastro-intestinal tract.
After preparation at pH 11 (above the IEP, isoelectric point, of the two proteins), the -rather strong- gels, remained locally folded and showed compact conformations, resembling the ones of the native proteins. After preparation at pH 8 (below the IEP of napin but above the one of cruciferin), the gels were softer, with SANS showing almost unfolded local structures. pH 8 could induce some destabilization of the conformations due to differences in the charges of the two proteins.
Under both gastric and intestinal digestion, differences are maintained. In gastric digestion, while a slight continuous decrease of the moduli was observed for the pH 8 gels, an unexpected increase in elasticity occurred for the pH 11 gels. It can be due to a competition between (i) unfolding, which increases the local interactions (and thus the modulus), and (ii) enzymatic scission of the protein bonds and crosslinks, which lowers the gel elasticity.
In the intestinal digestion step, rheological differences of the two gels tended to attenuate: moduli of both gels decrease progressively, indicating loss of connectivity at larger size. Strikingly, proteins of both gels experience a re-compaction, which may have consequences on the bio-availability. For longer times than studied with SANS, rheology suggests that intestinal digestion proceeds via some erosion of the gel.
These results have been confirmed with very nice accuracy and systematicity by recent SAXS measurements during the SLAMM-BAG on SWING by z-scanning of a kinetic gradient of digestion along capillaries created by digestive juices poured on top, at successive times t. We can follow in great detail the back-and-forth evolution of the conformation (manuscript to be submitted), over a (z,t) master curve.
Monitoring food structure in plant protein gels during digestion: rheometry and Small Angle Neutron Scattering studies M. Napieraj, A. Brûlet, E. Lutton, U. Randrianarisoa, A. Boire, F. Boué Food Structure, vol.32, 100270, 2022 https://doi.org/10.1016/j.foostr.2022.100270